24 june 2025
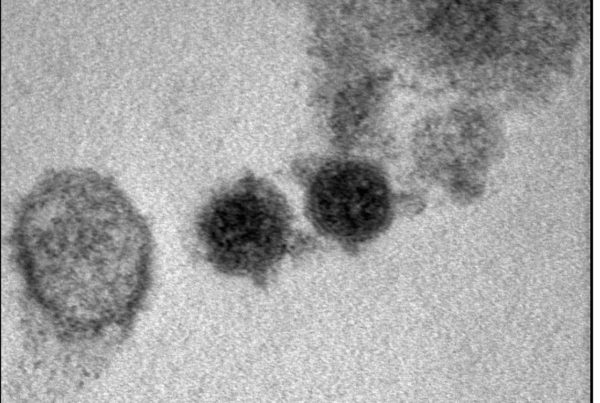
Genoscience Pharma bénéficie d’un financement européen pour développer un traitement novateur contre la fibrose pulmonaire idiopathique
Marseille-24 Juin 2025 – Genoscience Pharma, entreprise de biotechnologie spécialisée dans le développement de traitements…
Read More