Since December 2019, SARS-CoV-2 has spread quickly worldwide, leading to more than
280 million confirmed cases, including over 5,000,000 deaths. Interestingly, coronaviruses were found
to subvert and hijack autophagic process to allow their viral replication. Autophagy-modulating
compounds thus rapidly emerged as an attractive strategy to fight SARS-CoV-2 infection, including
the well-known chloroquine (CQ). Here, we investigated the antiviral activity and associated mechanism
of GNS561/Ezurpimtrostat, a small lysosomotropic molecule inhibitor of late-stage autophagy.
Interestingly, GNS561 exhibited antiviral activity of 6–40 nM depending on the viral strain considered,
currently positioning it as the most powerful molecule investigated in SARS-CoV-2 infection. We then
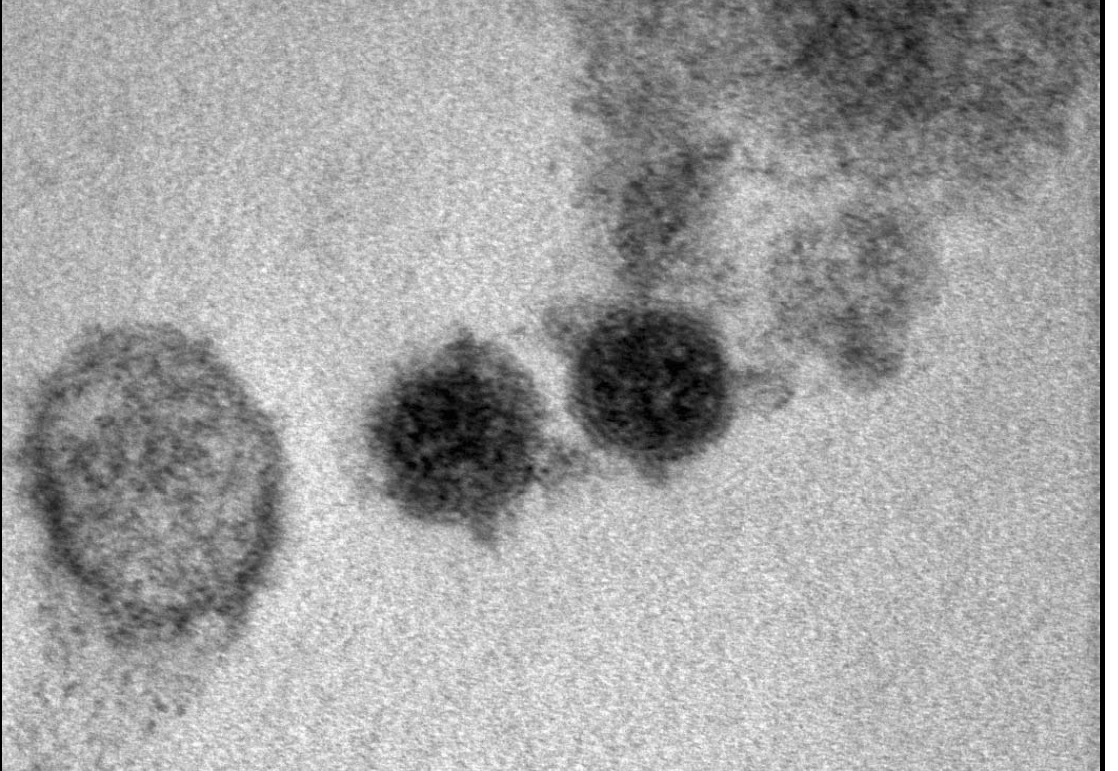
showed that GNS561 was located in lysosome-associated-membrane-protein-2-positive (LAMP2-
positive) lysosomes, together with SARS-CoV-2. Moreover, GNS561 increased LC3-II spot size and
caused the accumulation of autophagic vacuoles and the presence of multilamellar bodies, suggesting
that GNS561 disrupted the autophagy mechanism. To confirm our findings, we used the K18-hACE2
mouse model and highlighted that GNS561 treatment led to a decline in SARS-CoV-2 virions in
the lungs associated with a disruption of the autophagy pathway. Overall, our study highlights
GNS561 as a powerful drug in the treatment of SARS-CoV-2 infection and supports the hypothesis
that autophagy blockers could be an alternative strategy for COVID-19.
You have a question ?