19 April 2023
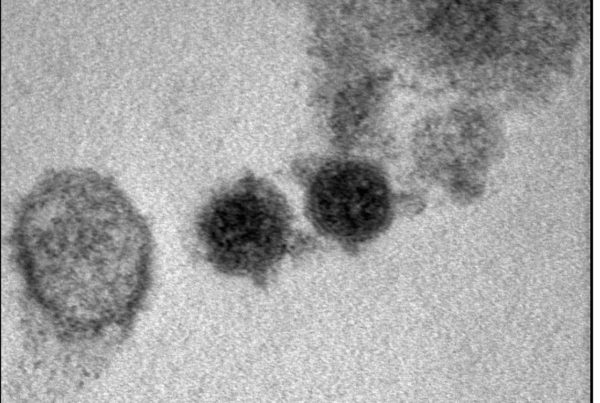
Genoscience Pharma has announced a new agreement for research at the Hebrew University of Jerusalem on targeted degradation technology
MARSEILLE-JERUSALEM, April 19, 2023 – Genoscience Pharma a clinical-stage biopharmaceutical company that develops disruptive therapeutics…
Read More